Lunit to present two abstracts about its AI-based tissue analysis platform Lunit SCOPE
Evidence supports the effectiveness of Lunit’s AI-powered tumor purity assessment method using Lunit SCOPE IO
Lunit announced its presentation of two abstracts featuring its AI-based immunotherapy response predictor at the 2022 United States and Canadian Academy of Pathology (USCAP) Annual Meeting, held in a hybrid, online-offline format on March 19-24.
Top iTechnology 5G News: ThunderSoft Joins With Partner Introduced the World’s First 5G End-to-end Multi-slicing Solution
As a leading medical AI provider, Lunit focuses on developing novel AI biomarkers for application in cancer treatment. Since 2019, the company has presented groundbreaking findings using its AI-powered tissue analysis platform, ‘Lunit SCOPE.’ Through digital analysis of histopathological whole slide images (WSI), the studies have validated the predictive value of Lunit SCOPE in immunotherapy.
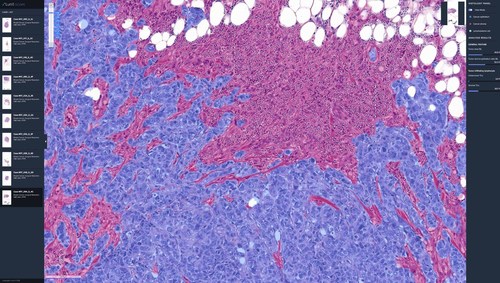
One of the USCAP abstracts showcases Lunit’s AI-powered tumor purity (AI-P) assessment method using the company’s AI biomarker platform, Lunit SCOPE IO–part of the Lunit SCOPE product line. Tumor purity is the proportion of cancer cells within a tissue sample and is estimated to ensure quality-controlled prediction of treatment effectiveness and prognosis. Findings from this study indicate that AI-P provides objective tumor purity quantification, validated through the consensus purity estimation based on next-generation sequencing (NGS) and pathological examination.
While advanced genomic analysis using NGS allowed for greater precision in estimating tumor purity compared to pathologic examination, direct evaluation of tumor purity assessment in a whole slide image (WSI) still faced limitations due to technical challenges. Clinical relevance between AI-P and existing tumor purity assessment methods indicate that AI-P can be used as a successful alternative to practically and expeditiously assess the tumor purity from H&E slides.
“This is our first study that validates AI-based tumor purity estimation,” said Brandon Suh, CEO of Lunit. “The results reveal AI’s capability to objectively analyze whole slide images compared to existing methods. Tumor purity serves as an important quality control element in NGS analysis, and an accurate assessment of tumor purity through AI can lead to increasingly accurate and cost-effective sequencing tests.”
Top iTechnology 5G News: ThunderSoft Joins With Partner Introduced the World’s First 5G End-to-end Multi-slicing Solution
The company’s other major study assessed the practical efficacy of Lunit SCOPE PD-L1, an AI-powered PD-L1 tumor proportion score (TPS) analyzer. According to the study, assistance with Lunit SCOPE PD-L1 substantially improved pathologists’ consensus and prediction of clinical response to immunotherapy in advanced non-small cell lung cancer (NSCLC). These results ultimately demonstrate the AI model’s effectiveness in helping to decide the most appropriate treatment for cancer patients.
Three board-certified pathologists manually evaluated the PD-L1 TPS of 479 NSCLC data, concordantly scoring in 81.4% of the cases. Following a revision of these evaluations based on AI suggestions, this overall consistency rate increased to 90.2%. Furthermore, the revised TPS group with AI assistance predicted objective response rate, progression-free survival, and overall survival better than the baseline group without AI assistance.
“While PD-L1 expression is the current standard for clinical application, manual evaluation of PD-L1 TPS by pathologists has practical limitations of interobserver bias and intensive labor,” said Chan-Young Ock, Chief Medical Officer at Lunit. “Through this study, we aimed to explore whether the AI-powered TPS analyzer could reduce the interobserver variation and increase the accuracy of analyses.”
Top iTechnology Cloud News: UJET Accelerates International Expansion and Announces Key New Hires
[To share your insights with us, please write to sghosh@martechseries.com]